Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fujita, Yoshitaka; Niizeki, Tomotake*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; Yamauchi, Yusuke*; Malgras, V.*; Kaneti, Y. V.*; Liu, C.-H.*; Hatano, Kentaro*; Suematsu, Hisayuki*; et al.
Bulletin of the Chemical Society of Japan, 95(1), p.129 - 137, 2022/01
Times Cited Count:8 Percentile:76.16(Chemistry, Multidisciplinary)In this work, the mechanisms responsible for the adsorption of molybdate ions on alumina are investigated using in-depth surface analyses carried out on alumina specimens immersed in solutions containing different molybdate ions at different pH values. The obtained results reveal that when alumina is immersed in an acidic solution containing molybdate ions, the hydroxyl groups present on the surface are removed to generate positively charged sites, and molybdate ions (MoO
 or AlMo
or AlMo O
O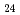 H
H
 ) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo
) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo O
O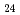 H
H
 , which is more easily desorbed than MoO
, which is more easily desorbed than MoO
 . Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
. Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
 ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope (
ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope ( Mo/
Mo/ Tc) generators.
Tc) generators.
 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:14.02(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
Tanaka, Tadao; Ogawa, Hiromichi; Muraoka, Susumu
Materials Research Society Symposium Proceedings, Vol.663, p.1169 - 1177, 2001/00
no abstracts in English
Tanaka, Tadao; Ogawa, Hiromichi
Proceedings of the International Workshop on Distribution and Speciation of Radionuclides in the Environment, p.278 - 284, 2000/00
no abstracts in English
 Np and
Np and  Am in sedimentary geological formation
Am in sedimentary geological formationTanaka, Tadao; Takebe, Shinichi; Ogawa, Hiromichi; Muraoka, Susumu
JAERI-Research 98-018, 20 Pages, 1998/03
no abstracts in English
Tanaka, Tadao; Ogawa, Hiromichi
JAERI-Conf 99-004, p.654 - 661, 1998/03
no abstracts in English
*; Nagasaki, Shinya*; *; Tanaka, Satoru*; Suzuki, Atsuyuki*; Tanaka, Tadao; Muraoka, Susumu
Radiochimica Acta, 82, p.239 - 242, 1998/00
no abstracts in English
 1 surface oxidation at room temperature
1 surface oxidation at room temperatureYoshigoe, Akitaka; Tsuda, Yasutaka; Tominaga, Aki; Sakamoto, Tetsuya; Ogawa, Shuichi*; Takakuwa, Yuji*
no journal, ,
The basic understanding of the oxidation at Si surfaces by oxygen gas has been important to develop advanced metal-oxide-semiconductor devices. In our previous study, we succeeded to detect a satellite peak located at lower binding energy sides of the main peak (Si-O-Si) in the Si(001)2 1 surface oxidation. This peak has been considered to be due to the molecularly adsorbed oxygen. In this study, we studied the incident O
1 surface oxidation. This peak has been considered to be due to the molecularly adsorbed oxygen. In this study, we studied the incident O energy dependence of this adsorbate by using real-time photoemission spectroscopy. All experiments were conducted at the surface experimental station (SUREAC2000) at BL23SU in SPring-8. It was found that the satellite peak was clearly observed. This result also implies the existence of a precursor state of adsorbed oxygen dissociation even for 0.06 eV.
energy dependence of this adsorbate by using real-time photoemission spectroscopy. All experiments were conducted at the surface experimental station (SUREAC2000) at BL23SU in SPring-8. It was found that the satellite peak was clearly observed. This result also implies the existence of a precursor state of adsorbed oxygen dissociation even for 0.06 eV.
Sumiya, Masatomo*; Tsuda, Yasutaka; Sumita, Masato*; Yoshigoe, Akitaka
no journal, ,
Synchrotron radiation X-ray photoelectron spectroscopy (XPS) was employed to clarify the oxidized states on the polar and m-plane GaN surfaces under exposure of various oxidation gases of H O, O
O, O , N
, N O, and NO. It was found that H
O, and NO. It was found that H O vapor has the higher reactivity. The oxygen was hardly adsorbed on the surface by irradiating N
O vapor has the higher reactivity. The oxygen was hardly adsorbed on the surface by irradiating N O and NO gases. Apparently, two oxidation states for O
O and NO gases. Apparently, two oxidation states for O and H
and H O irradiation were detected on +c GaN surface. Physisorption of O
O irradiation were detected on +c GaN surface. Physisorption of O molecule was dominate. The dissociation and adsorption of H
molecule was dominate. The dissociation and adsorption of H O molecules co-existed on the +c surface. The chemisorption on the m-plane of GaN was dominant, and a stable Ga-O bond was formed on the surface. These chemical oxygen states were simulated by density functional molecular dynamics calculation using a theoretical model including both electronic spins on the surfaces and the polarity of GaN.
O molecules co-existed on the +c surface. The chemisorption on the m-plane of GaN was dominant, and a stable Ga-O bond was formed on the surface. These chemical oxygen states were simulated by density functional molecular dynamics calculation using a theoretical model including both electronic spins on the surfaces and the polarity of GaN.